Submitted by L. Millard on Wed, 24/11/2021 - 10:19
Glioblastoma (GB) is the most common primary brain cancer in adults and has one of the lowest median survival rates for cancer of just 4.6 months when left untreated. Even patients who meet the criteria to undergo the current standard treatment – surgery to remove the tumour followed by radiotherapy and chemotherapy – still only experience a median survival of approximately 14 months. There is pressing need for improved treatment methodologies.
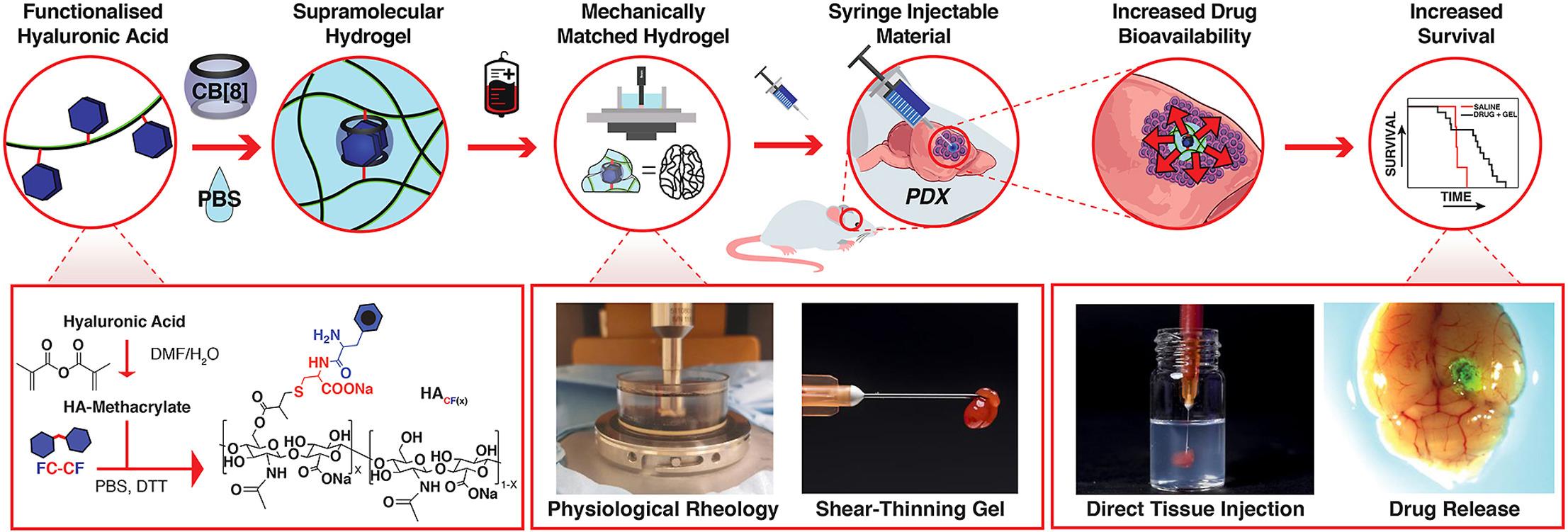
A collaborative research study exploring the potential of a hydrogel drug delivery approach has been published in the international journal Biomaterials. The study, and published paper titled ‘Mechanically matching the rheological properties of brain tissue for drug-delivery in human glioblastoma models’, features members of the IRC, Professors Oren Scherman, Richard Gilbertson and Colin Watts, and researchers from the Melville Laboratory for Polymer Synthesis in the Department of Chemistry and Department of Clinical Neurosciences Centre for Brain Repair at the University of Cambridge. The research brings focus to the development of a new class of drug delivery reservoirs consisting of peptide-functionalised hyaluronic acid (HACF) dynamically cross-linked by the macrocycle cucurbit[8]uril (CB[8]).
The study measured the mechanical and viscoelastic properties of native human and mouse tissues under physiological conditions. Hydrogels were then formulated with matching properties with the aim of improving drug bioavailability for glioblastoma tumours in patient-derived xenograft (PDX) models. Increased survival in PDX mouse models was observed following the use of an injectable drug-loaded hydrogel which can be tuned to match the mechanical properties of native brain tissue from human or mouse.
As well as clearly signalling the potential of biocompatible hydrogels for localised treatment of re-sectable glioblastomas, the paper also highlighted areas of future study. Two different PDX models were developed using seeding cells from either the core or margin of a GB tumour. These resulted in PDX tumours with very different properties. Further exploration is needed to fully understand the implications of these differences.
Additionally, further work is planned to better understand how closely the hydrogels need to match the mechanical properties of the surrounding tissue for efficacious drug delivery. The rheological measurements conducted within the study and the ability to tailor the hydrogels are the start of this process.
The use of biocompatible and tailorable materials to target drug delivery for GB could be significant in helping to overcome barriers to treatment such as the stiffness of tumour tissue, which can prevent drugs reaching the target and cause increased pressure in the brain. While this study saw a significant survival advantage in one of the PDX mouse models after drug-loaded hydrogel injections, it also observed a significant number of surgery-associated deaths in mice with a stiffer tumour model linked to the infiltrative nature of these tumours. The study concluded there is a need for future studies including GB tumour resection to more accurately represent and assess true clinical translation.
• Christopher C. Parkins, Joseph H. McAbee, Lisa Ruff, Astrid Wendler, Richard Mair, Richard J. Gilbertson, Colin Watts, Oren A. Scherman, ‘Mechanically matching the rheological properties of brain tissue for drug-delivery in human glioblastoma models’, Biomaterials, Volume 276, 2021,120919, ISSN 0142-9612, https://doi.org/10.1016/j.biomaterials.2021.120919
• Visual courtesy of Dr Chris Parkins


