Submitted by L. Millard on Thu, 10/11/2022 - 09:52
Professor Oren Scherman has received an MRC Confidence in Concept (CiC) award aimed at acceleration of the translational pathway of a promising invention.
The CiC award has been granted to Prof Scherman, Director of the Melville Laboratory and Professor of Supramolecular and Polymer Chemistry in the Yusuf Hamied Department of Chemistry at the University of Cambridge, to support the next stage in development of a supramolecular local drug delivery hydrogel depot for glioblastoma (GB), one of the three hard-to-treat cancers in sight of the IRC programme.
We have made tremendous progress so far in the IRC on the development of the hydrogel technology. I'm delighted that we have secured the MRC Confidence in Concept grant. This funding will enable us to meet the next milestones in translating our technology to clinical use Professor Oren Scherman, IRC Investigator
GB is the most common high-grade primary brain tumour in adults with one of the lowest median survival rates of just 3 months if left untreated. It presents significant challenges to treatment on account of poor drug penetration of the Blood-Brain-Barrier meaning systemic chemotherapy has very little effect and the highly infiltrative nature of GB means it is recurrent in approximately 90% of patients. Patient outcomes remain poor despite decades of research, illustrated by a median survival of just 14.4 months. When surgery to remove the tumour is possible, complete removal is hampered by where and how the tumour infiltrates the brain. Surgery is followed by a combination of radiotherapy and chemotherapy.
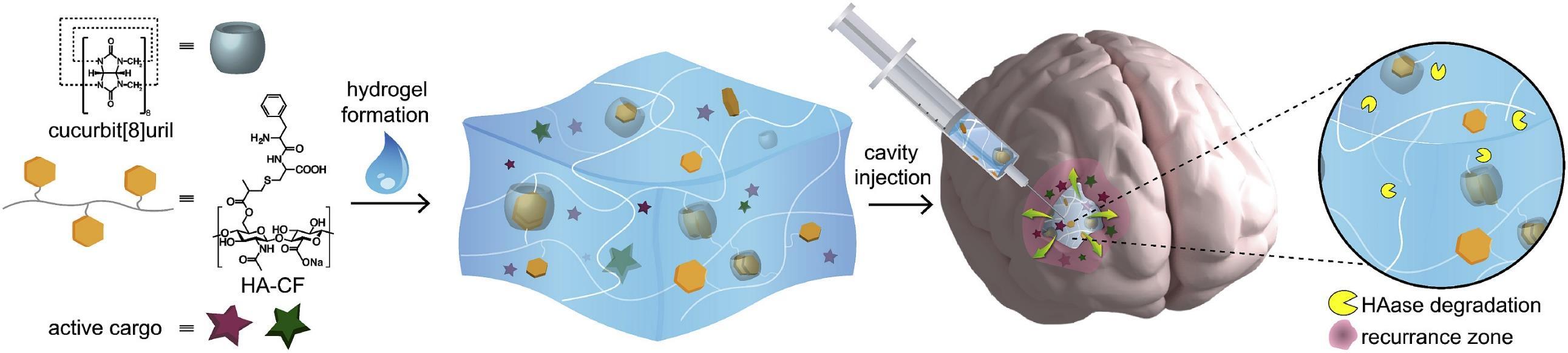
Over the last decade Professor Scherman and his research group have developed a biocompatible and biodegradable hydrogel for drug delivery by repurposing existing chemotherapeutics.1,2 The drug-loaded hydrogel is viscous and can be applied by a neurosurgeon via surgical syringe to cavity walls to target remaining GB cells following resection. This treatment could increase the life span of patients with recurrent GB – a patient group for whom no standard of care currently exists.
Prof. Scherman said: “We have made tremendous progress so far in the IRC on the development of the hydrogel technology. I'm delighted that we have secured the MRC Confidence in Concept grant. This funding will enable us to meet the next milestones in translating our technology to clinical use.”
The hydrogel technology has been tested in a range of both in vitro and in vivo experiments and demonstrated to be safe to use in mice. There was a substantial increase in lifespan, using a patient-derived xenograft (PDX) model when mice were treated with drug-loaded hydrogels.2 With the primary aim of progressing the technology towards a first-in-human clinical trial, the hydrogel has reached a translational milestone to establish a maximum tolerated dose (MTD) of a therapeutic drug.
Dr Beatrix Kiddle, Translational Research Project Manager at the Office for Translational Research at the University of Cambridge, supported the application for the MRC CiC funding award. Together with IRC collaborators from the University of Nottingham, Drs Ruman Rahman and Stuart Smith, the aim is to determine a MTD and study its therapeutic effect (2). If successful in showing that GB can be effectively treated in rats with drug-loaded hydrogel in vivo, the translation of the hydrogel technology can progress toward Absorption, Distribution, Metabolism and Excretion (ADME)-toxicology studies in agreement with the Medicines and Healthcare products Regulatory Agency (MHRA), bringing the technology closer to a clinical trial.
• Image caption
Schematic overview of supramolecular hydrogel developed by Prof Scherman for local delivery towards GB following resection.
• References
- Rowland et al, 2018 https://doi.org/10.1016/j.biomaterials.2018.05.054
- Parkins et al, 2021 https://doi.org/10.1016/j.biomaterials.2021.120919
- Smith et al, 2019, https://doi.org/10.1158/1078-0432.CCR-18-3850


